

As Canadian doctors wait for a decision on antivirals, U.S. counterparts wonder where the supply is
While Canada has some of the highest vaccination rates in the world — a development that has dramatically reduced cases of severe illness — infections among the unvaccinated and breakthrough cases in those with two doses are still testing a health-care system that is on the ropes after two years of the pandemic.
But there has been no movement yet on federal authorization of two antiviral treatments that have been approved in some other Western jurisdictions — Paxlovid by Pfizer and Merck’s molnupiravir. The Canadian government has struck deals for supplies of both, pending approval.
Dr. Zain Chagla, associate professor at McMaster University in Hamilton and an infectious diseases physician, says therapeutics — especially those like Paxlovid that can be administered outside a hospital setting — are “absolutely” a “game-changer.”
“We know that vaccines have an incredible role to play but we need a backup option,” Chagla told CBC News. “Therapeutics give the highest-risk people the chance to stay out of hospital and have a benign recovery like everybody else.”
It has been reported that in a phone call earlier this week, some premiers were champing at the bit, urging Prime Minister Justin Trudeau to make antiviral procurement a top priority.
The best Health Minister Jean-Yves Duclos could tell reporters on Wednesday was that a decision by Health Canada would be “very soon,” an answer not substantially different than when federal health officials were pressed on the topic before Christmas.
Kevin Smith, the CEO of the University Health Network in Toronto, went public with demands for Paxlovid’s approval in early January, calling it an “essential addition” to hospitals’ needs. While he said he has respect for the regulatory process at Health Canada, he struggles to understand why the United States and United Kingdom approved the drugs much more quickly than Canada.
“My problem is, at the moment, the perception of the clinical community who are struggling, greatly struggling, is we don’t have approval for this drug,” he said in an interview with The Canadian Press.
In an interview with The Canadian Press published Thursday, federal government chief medical adviser Dr. Supriya Sharma said Health Canada should be ready to make an authorization decision about Paxlovid in a week to 10 days. The questions about molnupiravir are more complicated, she said. (As explained in a previous newsletter, the Pfizer product appears more effective than Merck’s based on clinical trials)
Both Duclos at the briefing and a Pfizer spokesperson in an email to CBC News said the expectation is that delivery would commence expeditiously after a government approval.
But the rub is that struggling American hospitals are having a difficult time obtaining the antivirals, according to multiple reports in U.S. media this week.
“Nobody’s seen it,” a doctor who treats patients at five hospitals in El Paso, Texas, said of Paxlovid while speaking to health news website Stat. “I tried to write a prescription for it and the local pharmacists have said, ‘We don’t have it. You can write all the prescriptions you want, but it doesn’t matter.'”
That means Canada, without manufacturing capacity at this point, might have to wait in line in order to get large enough quantities for Paxlovid to qualify as any kind of game-changer. And most certainly not to address the early effects of Omicron, which federal modelling released Friday suggested could lead to a further surge soon.
Meanwhile, a World Health Organization panel on Thursday strongly recommended Eli Lilly’s baricitinib, sold under brand name Olumiant, for patients with severe COVID-19 in combination with corticosteroids. The WHO guidelines, published in the British Medical Journal, noted that evidence shows baricitinib improves survival rate and reduces the need for ventilation, with no observed increase in adverse effects.
Baricitinib, an oral medication, has been previously approved by Health Canada for its original purpose, the treatment of rheumatoid arthritis. The Ontario Science Table, among others in Canada, have released guidelines that recommend its use under some conditions for moderately or critically ill COVID-19 patients.
From The National
The At Issue panel discusses Quebec’s proposed tax on adults who choose to not receive the COVID-19 vaccine. Plus, the panellists look at whether health-care funding in Canada will change after the pandemic. 12:48
B.C. officials cautiously optimistic cases may have peaked, but not so for hospitalizations
Modelling shows community spread of the highly transmissible Omicron variant is on the decline, the B.C. government says, but the province is not out of the woods yet.
On Thursday, B.C. reported that 534 people were in hospital with COVID-19, the highest number at any point in the pandemic. Of those, 102 were in intensive care.
The province also reported seven more deaths from the disease and 2,554 new cases.
As with other provinces, the variant has been responsible for staffing shortages in many sectors, including among police, paramedic and health-care workers. As well, B.C.’s largest provider of medical laboratory services, LifeLabs, has also been forced to close or reduce hours at 16 locations for at least two weeks because of staffing issues related to the variant.
Provincial Health Officer Bonnie Henry said at a briefing on Friday that while the Omicron variant is dominant, Delta variant cases still exist in the province. She said that of COVID-19 hospitalizations, about 45 per cent are incidental positive cases related to admissions for other reasons.
Henry says community transmission has decreased in the Vancouver Coastal Health, Fraser Health and Island Health regions so far, but there is still likely to be a rise in hospitalizations in the coming week or two.
This, according to the province, is because of a lag time between peak community transmission and peak hospitalizations during the Omicron wave as evidenced in other parts of the world, such as New York City and London, where the variant arrived earlier.
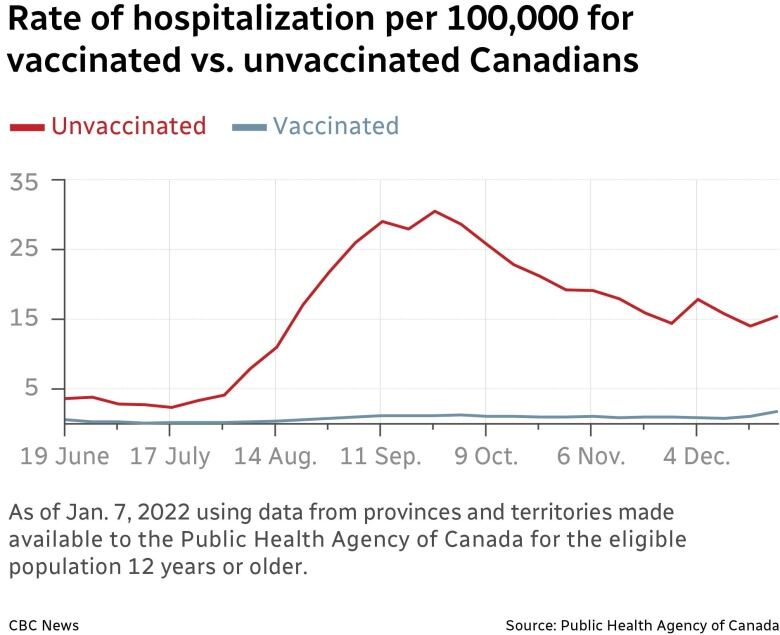
Health Minister Adrian Dix said the response for booster doses has been positive overall, but he urged the estimated 53,000 people older than 70 who’ve yet to take up a booster to do so. Dix’s plea came after Henry presented findings that indicated the hospitalization rate was five times higher for the unvaccinated than the vaccinated.
FIRST PERSON
This is what a 16-hour hospital shift is like for me while Omicron rages
This First Person column is the experience of Laura Sang, a resident physician at a hospital in Montreal.
“Despite being entrenched in the front lines of the pandemic for two years, this wave feels different,” Sang writes of the “sheer volume” of patients being seen at her hospital.
For more information about CBC’s First Person stories, please see the FAQ.
How many vaccine doses do we need to stay a step ahead of the pandemic?
Immunocompromised Ontarians were able to receive their fourth COVID-19 vaccine doses beginning Friday, as long as they were at least 84 days past their third dose.
The province is among a few that have already started administering fourth doses in long-term care homes, retirement homes and other congregate settings.
“Many of these individuals are now likely becoming increasingly more susceptible to COVID-19 infection due to waning immunity from their previous doses,” Chief Medical Officer of Health Dr. Kieran Moore said at a briefing Thursday in explaining the limited push, which was based on advice from the province’s vaccine advisory committee.
Discussions about fourth COVID-19 shots are already playing out in the public sphere, and as has happened before during the pandemic, Israel has been among the most aggressive in its approach.
Israel began administering second boosters to the most vulnerable late last month and later began offering them to everyone older than 60. Prime Minister Naftali Bennett signed off on the second booster after preliminary findings of an Israeli study that indicated a fourth shot could boost antibodies five-fold a week after the shot is administered.
There’s a delicate balance to be struck in offering protections at the individual level as compared to protections at the population level, and how much can be asked of citizens, Dr. Isaac Bogoch told CBC’s The Dose this week.
“Vaccinating people every four months, or every six months, is obviously not a very sustainable or smart approach,” said Bogoch, an infectious diseases specialist at Toronto General Hospital.
There is skepticism as to whether Israel’s approach is necessary right now in Europe and North America. U.S. infectious diseases expert Dr. Anthony Fauci said recently federal health authorities there are in favour of a more gradual approach.
“It is conceivable that in the future, we might need an additional shot, but right now, we are hoping that we will get a greater degree of durability of protection from that booster shot,” Fauci said.
The European Union’s drug regulator on Tuesday expressed doubts about the need for a fourth booster dose of COVID-19 vaccine and said there is currently no data to support this approach.
“While use of additional boosters can be part of contingency plans, repeated vaccinations within short intervals would not represent a sustainable long-term strategy,” the European Medicines Agency’s head of vaccines strategy, Marco Cavaleri, told a media briefing.
British health officials last week said it’s status quo for now, given that third shots are providing high levels of protection for older people against severe disease.
To Bogoch, it’s likely Canada is at the stage in the pandemic where the focus should be primarily on preventing the most severe disease and outcomes, as opposed to preventing the most infections.
There is an additional consideration, brought up frequently by the World Health Organization, activists and ethicists.
“Are you giving third, and of course fourth doses at the expense of many places on Earth that haven’t even given a first or a second dose? I think that’s an extremely important point to recognize,” said Bogoch.
Meanwhile, Israel presses on, noting Friday through its Health Ministry that 500,000 people there had received a fourth dose.
Today’s graphic:
Find out more about COVID-19
For full coverage of how your province or territory is responding to COVID-19, visit your local CBC News site.
To get this newsletter daily as an email, subscribe here.
See the answers to COVID-19 questions asked by CBC viewers and readers.
Still looking for more information on the pandemic? Reach out to us at [email protected] if you have any questions.








More Stories
Peguis First Nation launches $1B flood damages lawsuit against feds, province and 2 municipalities | CBC News
Cuba apologizes to Canada for delivering wrong body to grieving Montreal family | CBC News
Big O costs keep piling up: Quebec greenlights $40M budget for fire repairs | CBC News